
Researchers at UW Health in Madison, Wisconsin are starting their Covid vaccine experiments on children 6 months up to 4 years old. The Pfizer vaccine was just recently given EUA for children 5 – 11 years-old.
UW Health announced a phase 3 clinical trial of the Moderna vaccine in kids ages 6 months to 4 years old had filled up. The trial has been underway for two weeks now.
“We’ve had an overwhelming response from the Madison community,” said Dr. Bill Hartman. “So, we had way more people interested in the study than we had spots.”
The vaccine clinical trial, called KidCOVE, is being carried out at 79 locations across 13 states and will involve roughly 13,275 participants between the ages of 6 months and 11 years old in its entirety.
“It is humbling to know that 53 million doses have been administered to people in the U.S.,” Stéphane Bancel, chief executive officer of Moderna, said in a statement. “We are encouraged by the primary analysis of the Phase 3 COVE study of mRNA-1273 in adults ages 18 and above and this pediatric study will help us assess the potential safety and immunogenicity of our COVID-19 vaccine candidate in this important younger age population.”
The University of Wisconsin School of Medicine and Public Health stated they already have all the applicants they need for the latest experiment on children unlike when they tested on kids between 5 and 11. In a statement given on Monday, UW Health noted the data from that age group has been passed along to Moderna, which is currently reviewing it.
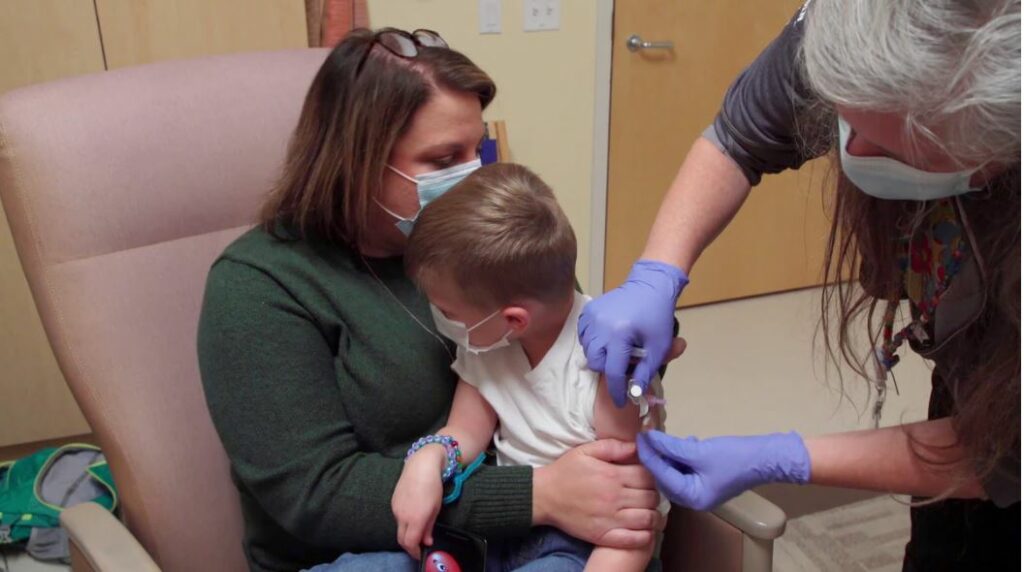
“This is the final frontier. Our very youngest children need to get the vaccine and we need to make sure they are safe,” UW–Madison’s co-principal investigator of the KidCOVE clinical trial Dr. Bill Hartman said. “The kids participating are heroes. They will be able to tell the story of how they helped save the world.”
Moderna plans to experiment on approximately 6,750 children throughout the United States and Canada in a two-part study. During part 1, participants 2 years to less than 12 years may receive one of two dose levels, while each participant ages six months to less than 2 years may receive one of three dose levels. This phase is already complete.
The second phase will use children between the ages of 2 years to less than 6 years old and they will undergo testing. The third and final stage will test the vaccine on children ages 6 months to less than 2 years old.
The trial is expected to last about 14 months and will be placebo-controlled. Parents and kids initially will not know which one they received.
Participants in the clinical trial are given two injections in the upper arm about 28 days apart and are then asked to return with their guardian to the study site for at least four follow-up appointments over the next 14 months, according to KidCOVE.
Is it such a good idea for parents to put their kids up as lab rats for these pharmaceutical companies? Even when we see reviews of vaccine studies on adults showing such vastly different results?
Should we be so willing to allow our children to be injected with such a highly experimental vaccine when we have no idea of the long-term side effects? Now is the time when we stand up to these corrupt companies and the tyrannical government and tell them no more. They will not use our children as their guinea pigs to see what will happen.
